.png)
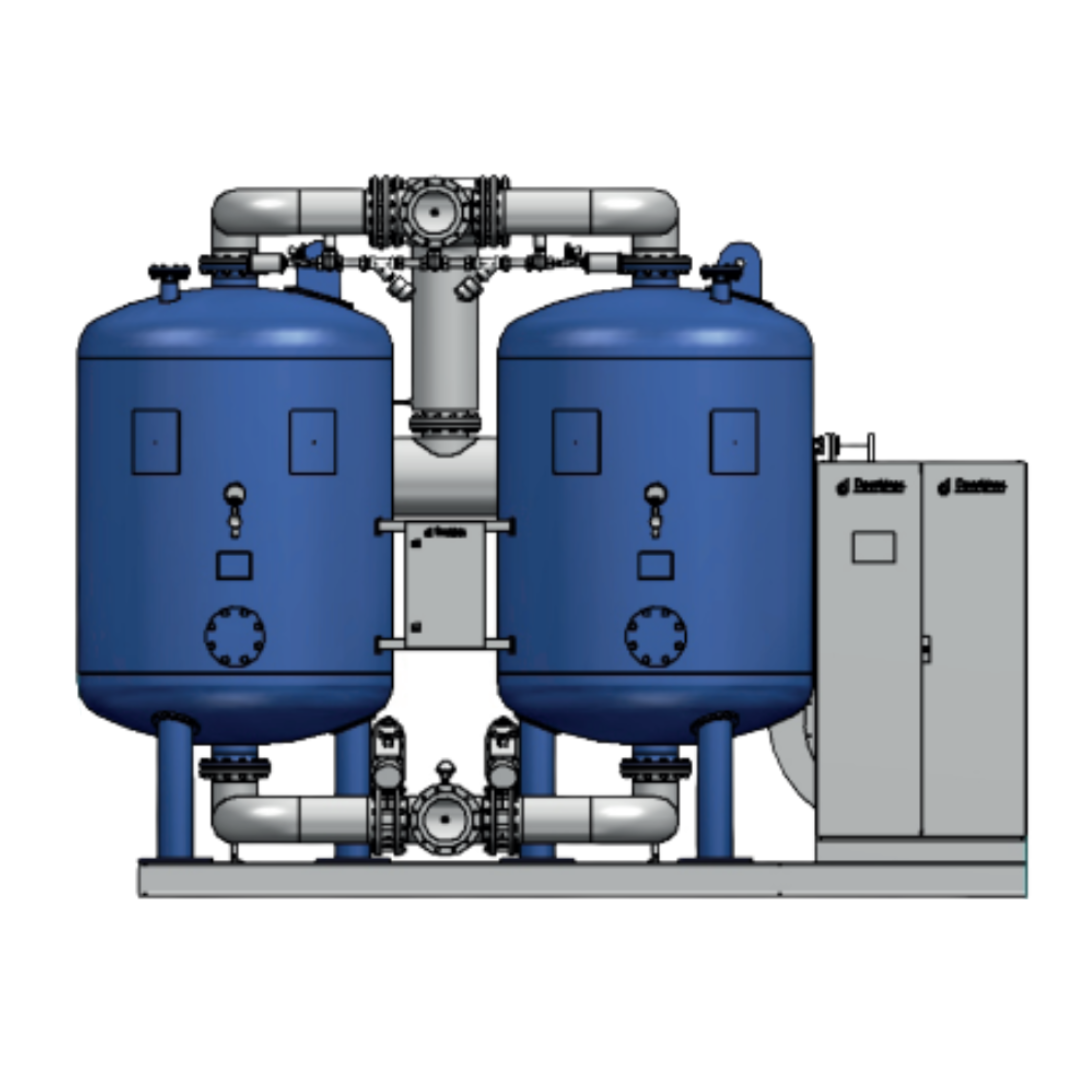
Stringent hygiene standards are set for production conditions to manufacture medicinal products, especially for clean room conditions. It is, therefore, crucial that production takes place in an environment free from germs, particles, bacteria, and contaminated oil. Compressed air is also highly required because it is often in direct contact with the product in the pharmaceutical industry's production process. Depending on the application, for example, compressed air must be sterile or oil-free. To fulfill the evidentiary obligation, e.g., for auditors, continuous monitoring (24/7) is required for compressed air in contact with the product.
Hygiene standards mean that each pharmaceutical company must determine which compressed air quality is appropriate and necessary for its production process.
Contact us now! We will be happy to assist you with determining the appropriate quality class and design of your compressed air treatment to keep the required compact air class constant and contribute to the reliability of your process.



























.png)



















.png)
.png)
.png)

.png)
.png)